Polymer Definition Chemistry: Understanding Macromolecular Structures
Introduction to Polymer Chemistry
Polymer chemistry represents a fundamental branch of macromolecular science that deals with the synthesis, characterization, and properties of polymer materials. Polymers are large molecules composed of repeating structural units connected by covalent chemical bonds. The term "polymer" originates from the Greek words "poly" meaning many and "meros" meaning parts, accurately describing these complex molecular structures.
In polymer definition chemistry, we study how small molecules called monomers combine through chemical reactions to form long chains or networks. These macromolecules exhibit unique physical and chemical properties that distinguish them from their monomeric precursors, making them invaluable in numerous industrial and biological applications.
Fundamental Concepts in Polymer Chemistry
Monomers and Polymerization
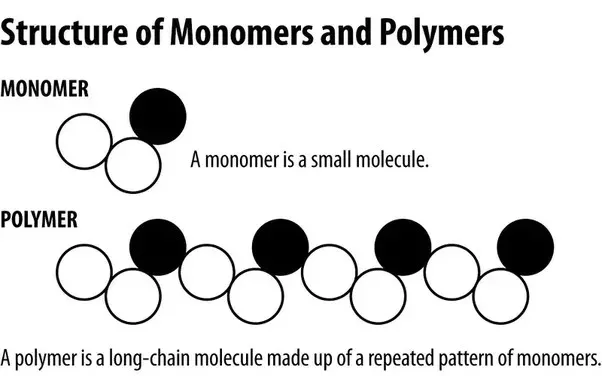
Monomers serve as the building blocks of polymers. These are relatively small molecules that contain reactive functional groups enabling them to form chemical bonds with other monomer molecules. The process by which monomers transform into polymers is called polymerization, which can occur through various mechanisms depending on the monomer type and reaction conditions.
Common examples of monomers include ethylene (C₂H₄), which polymerizes to form polyethylene, and styrene (C₆H₅CH=CH₂), which forms polystyrene. The chemical structure and properties of the monomer directly influence the characteristics of the resulting polymer.
Degree of Polymerization
The degree of polymerization (DP) represents the number of monomeric units in a polymer molecule. This crucial parameter significantly affects the polymer's physical properties, including melting point, tensile strength, and viscosity. Higher DP values generally correlate with improved mechanical properties but may also increase processing difficulties.
Classification of Polymers
Based on Source
Polymers can be categorized according to their origin into three main groups:
| Polymer Type | Definition | Examples |
|---|---|---|
| Natural Polymers | Occur naturally in plants and animals | Cellulose, proteins, natural rubber, DNA |
| Semi-synthetic Polymers | Chemically modified natural polymers | Rayon, cellulose acetate |
| Synthetic Polymers | Man-made through chemical synthesis | Polyethylene, nylon, polyester, PVC |
Based on Structure
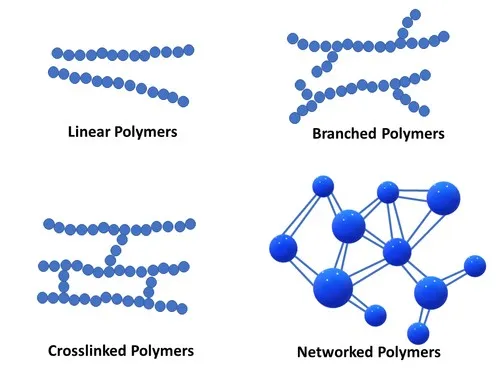
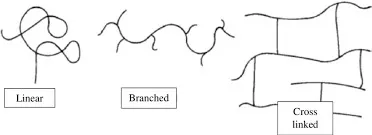
The molecular architecture of polymers significantly influences their properties and applications. Structural classifications include:
- Linear Polymers: Consist of long continuous chains without branches
- Branched Polymers: Contain side chains attached to the main backbone
- Cross-linked Polymers: Feature covalent bonds between polymer chains
- Network Polymers: Form three-dimensional networks with extensive cross-linking
Polymerization Mechanisms
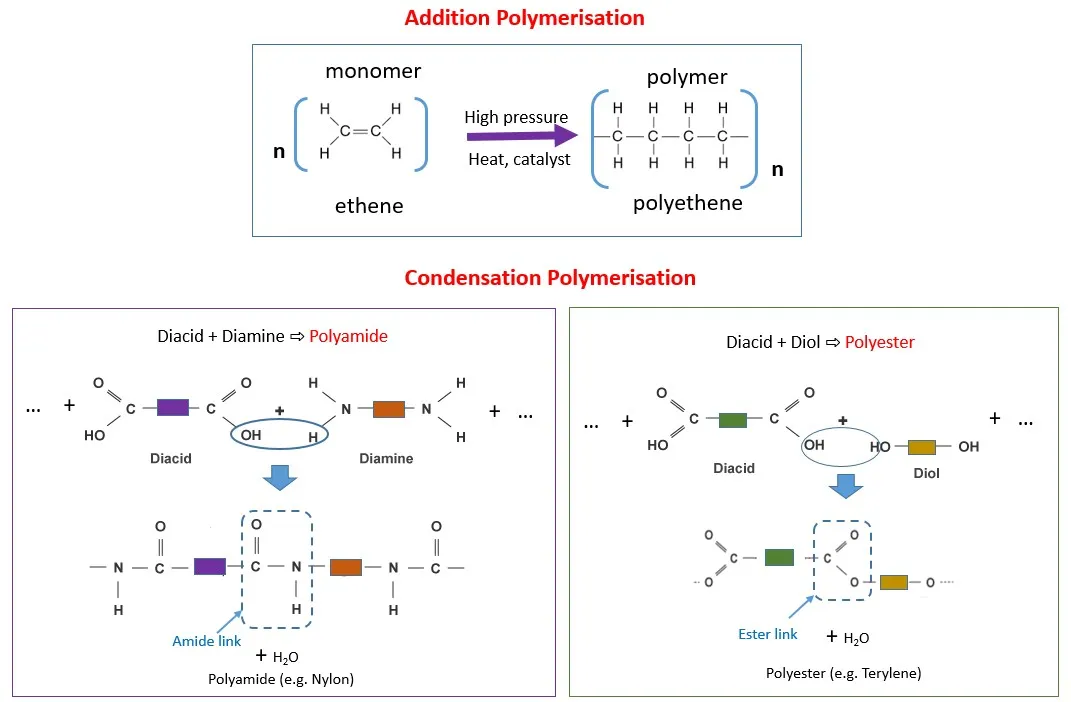
Addition Polymerization
Addition polymerization, also known as chain-growth polymerization, involves the sequential addition of monomer molecules to a growing polymer chain. This process typically requires an initiator to start the reaction and proceeds through three distinct stages: initiation, propagation, and termination.
The mechanism of addition polymerization can be further classified based on the reactive intermediate involved:
| Mechanism Type | Reactive Intermediate | Common Polymers |
|---|---|---|
| Free Radical | Radicals | Polyethylene, polystyrene, PVC |
| Cationic | Carbocations | Polyisobutylene |
| Anionic | Carbanions | Polybutadiene, some nylons |
Condensation Polymerization
Condensation polymerization, or step-growth polymerization, involves the reaction between bifunctional or polyfunctional monomers with the elimination of small molecules such as water, alcohol, or hydrogen chloride. This process typically occurs more slowly than addition polymerization and requires careful control of reaction conditions.
Common examples include the formation of polyesters from diols and diacids, and polyamides from diamines and diacids. The molecular weight of condensation polymers increases gradually throughout the reaction, unlike the rapid chain growth observed in addition polymerization.
Polymer Properties and Characterization
Molecular Weight Distribution
Unlike small molecules, polymers do not possess a single molecular weight but rather a distribution of molecular weights. This polydispersity index (PDI) significantly impacts the polymer's processing and end-use properties. Characterization techniques such as gel permeation chromatography (GPC) and light scattering are employed to determine molecular weight distributions.
Thermal Properties
Polymers exhibit distinctive thermal behaviors that determine their applications. Key thermal properties include:
- Glass Transition Temperature (Tg): The temperature at which amorphous polymers transition from glassy to rubbery state
- Melting Temperature (Tm): The temperature at which crystalline regions of polymers melt
- Thermal Degradation Temperature: The temperature at which polymer chains begin to decompose
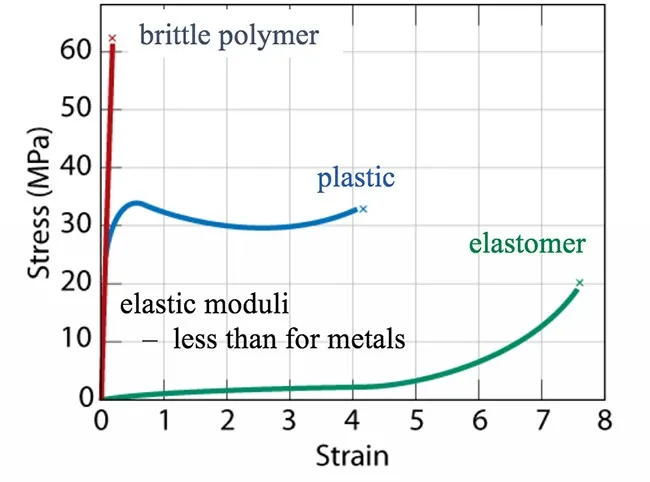
Mechanical Properties
The mechanical behavior of polymers encompasses characteristics such as tensile strength, elongation at break, modulus of elasticity, and impact resistance. These properties depend on factors including molecular weight, crystallinity, cross-linking density, and the presence of additives or fillers.
Applications of Polymers
Industrial and Commercial Applications
Polymers have revolutionized numerous industries due to their versatile properties and relatively low production costs. Major application areas include:
| Application Sector | Polymer Examples | Key Properties Utilized |
|---|---|---|
| Packaging | Polyethylene, polypropylene, PET | Barrier properties, flexibility, transparency |
| Automotive | Polyurethane, ABS, polycarbonate | Impact resistance, durability, lightweight |
| Electronics | Epoxy resins, silicones, polyimides | Electrical insulation, thermal stability |
| Textiles | Nylon, polyester, spandex | Strength, elasticity, dyeability |
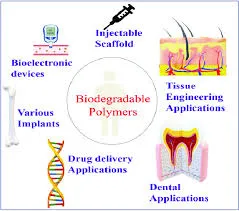
Biomedical Applications
In the biomedical field, polymers play crucial roles in drug delivery systems, tissue engineering scaffolds, medical devices, and surgical implants. Biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA) are particularly valuable for temporary medical applications where the polymer should safely degrade within the body.
Recent Advances in Polymer Chemistry
Smart and Responsive Polymers
Recent research has focused on developing "smart" polymers that respond to external stimuli such as temperature, pH, light, or specific chemical signals. These stimuli-responsive polymers find applications in controlled drug delivery, sensors, and self-healing materials.
Sustainable and Biodegradable Polymers
With growing environmental concerns, significant effort is directed toward developing sustainable polymers from renewable resources and designing biodegradable alternatives to conventional plastics. Bio-based polymers such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs) represent promising solutions to plastic pollution.
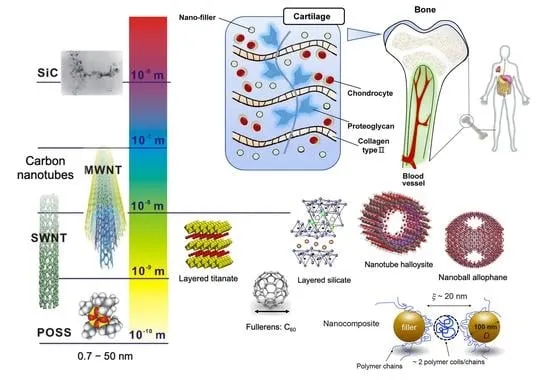
Advanced Polymer Nanocomposites
The incorporation of nanomaterials such as carbon nanotubes, graphene, and nanoclay into polymer matrices has led to the development of polymer nanocomposites with enhanced mechanical, thermal, and electrical properties. These advanced materials open new possibilities in aerospace, electronics, and energy storage applications.
Conclusion
Polymer definition chemistry provides the fundamental understanding necessary to design, synthesize, and characterize macromolecular materials with tailored properties for specific applications. From everyday plastics to advanced biomedical devices, polymers continue to transform technology and improve quality of life. As research progresses, we can expect continued innovation in polymer science, particularly in areas addressing sustainability, functionality, and performance enhancement.
The interdisciplinary nature of polymer chemistry ensures its continued relevance across multiple scientific and engineering disciplines, driving advancements that will shape future materials and technologies.

