Metallic materials form the backbone of modern engineering and technology. At their core, metallic materials are substances composed primarily of metal atoms, characterized by a unique set of physical and chemical properties arising from metallic bonding. This definition, while simple, unlocks a world of materials essential to everything from skyscrapers and automobiles to electronics and medical devices. Unlike ionic or covalent compounds, metals exhibit a lattice structure where valence electrons are delocalized, forming a "sea of electrons" that grants them their distinctive traits.
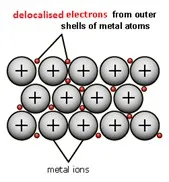
Visualization of the metallic bond: a regular lattice of positive metal ions surrounded by a cloud of delocalized electrons.
Fundamental Characteristics of Metallic Materials
The definition of a metallic material is operationalized through a suite of defining characteristics. These properties collectively distinguish metals from polymers, ceramics, and composites.
1. Metallic Bonding and Structure
The metallic bond is the foundational concept. Metal atoms release their outer electrons, which become shared collectively among all positive metal ions (cations). This structure is responsible for most metallic properties.
2. Key Physical and Mechanical Properties
Metals are renowned for their strength, durability, and functionality. Primary properties include:
- High Electrical and Thermal Conductivity: The free-moving electrons efficiently transport charge and heat energy.
- Malleability and Ductility: The non-directional bonding allows layers of atoms to slide past each other without fracturing, enabling shaping into sheets and wires.
- Metallic Luster: The interaction of electrons with light gives metals their characteristic shiny appearance.
- High Strength and Hardness: Though variable by alloy, metals generally resist deformation and indentation.
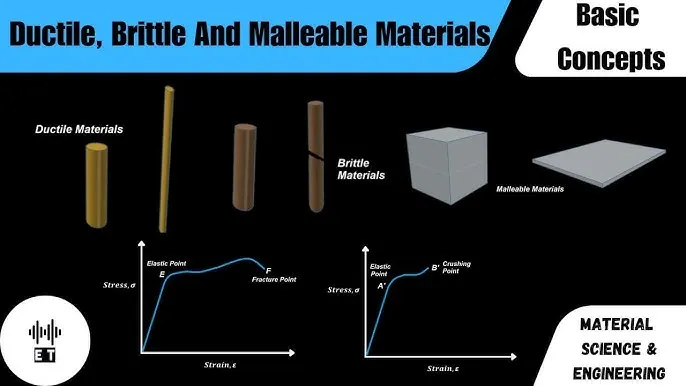
Demonstration of metallic malleability: a metal sheet being formed versus a brittle material cracking under similar force.
Classification of Metallic Materials
Metallic materials are not monolithic; they are systematically classified based on composition and iron content.
| Classification | Definition & Key Components | Common Examples | Primary Applications |
|---|---|---|---|
| Ferrous Metals | Metals where iron (Fe) is the principal constituent. They are known for high strength and magnetic properties, but often prone to corrosion without protection. | Carbon steels, Stainless steels, Cast Iron | Structural frameworks, automotive chassis, machinery, tools. |
| Non-Ferrous Metals | Metals containing little to no iron. They often offer advantages like corrosion resistance, lighter weight, and higher conductivity. | Aluminum, Copper, Zinc, Titanium, Gold | Aerospace components, electrical wiring, packaging, jewelry. |
| Alloys | Engineered materials composed of two or more metallic elements (or a metal and non-metal) to enhance specific properties. | Brass (Cu+Zn), Bronze (Cu+Sn), Steel (Fe+C), Duralumin (Al+Cu) | Specialized engineering, marine hardware, musical instruments, aircraft frames. |
The Role of Metallic Materials in Advanced Engineering
Moving beyond the basic definition, modern technology leverages advanced metallic materials. The development of superalloys, shape-memory alloys, and metallic glasses pushes the boundaries of performance in extreme environments, such as jet engines and medical implants. These materials are designed at the atomic level, showcasing how the fundamental principles of metallic bonding can be harnessed and refined.
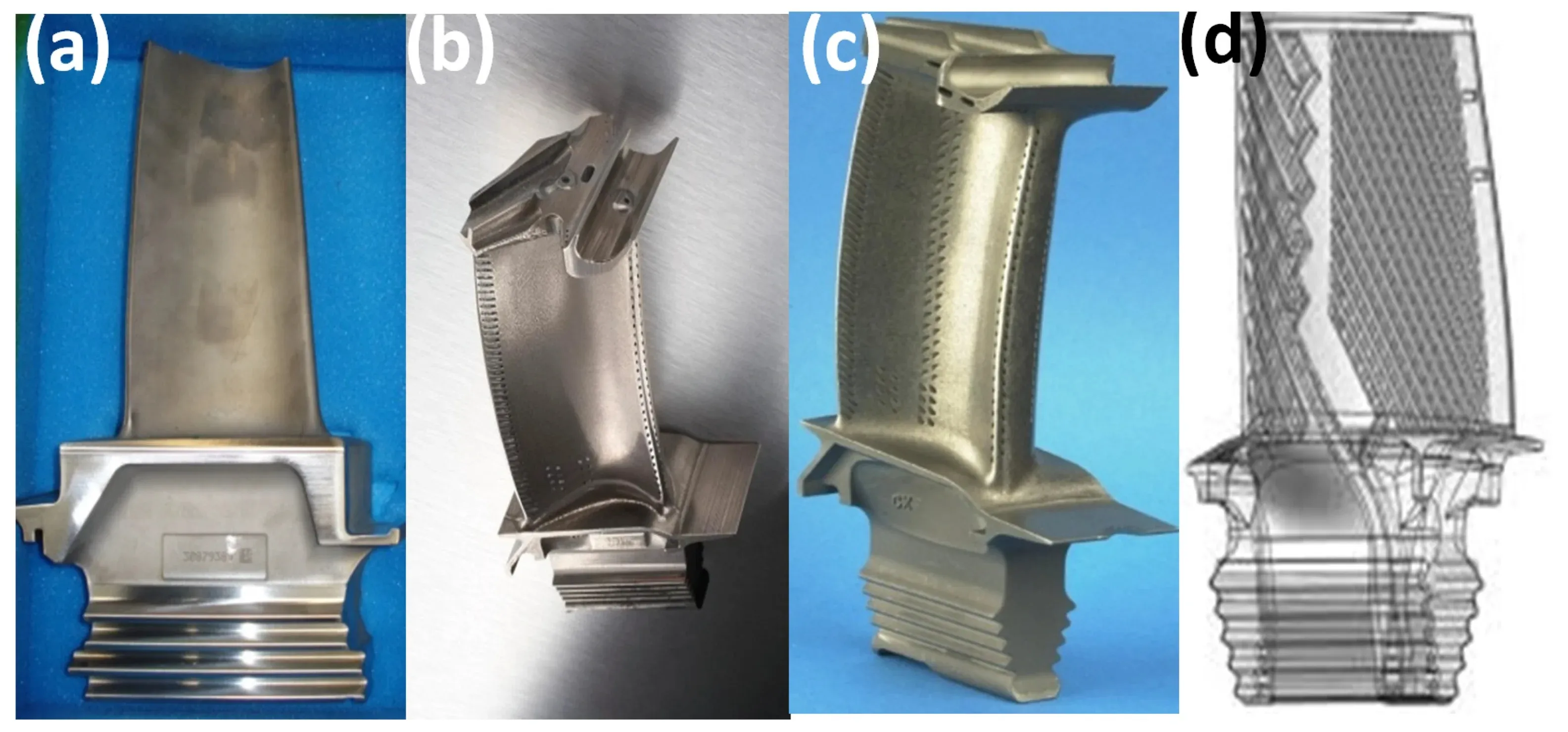
High-performance nickel-based superalloys in turbine blades and titanium alloys in biomedical implants.
Environmental and Future Considerations
The definition of metallic materials is now intertwined with sustainability. Research focuses on developing recyclable, low-energy production alloys and corrosion-resistant materials to extend product lifecycles. The future lies in smart metallic materials that can respond to environmental stimuli like stress or temperature change.
Conclusion
In summary, the definition of metallic materials encompasses substances defined by metallic bonding, leading to high conductivity, malleability, and strength. They are categorized into ferrous, non-ferrous, and alloy families, each serving critical roles across all industrial sectors. As material science advances, this definition expands to include sophisticated, multi-functional alloys that will drive future technological innovation, proving that metals remain as vital and dynamic today as at the dawn of the Bronze Age.

